Introduction
CROs need automated reporting capabilities to save time and reduce errors. Here’s how to achieve this need by leaning on Customer Success Team (CSM) to go beyond the standard software configuration.
Contract research organizations (CROs) face intense pressure to meet tight development timelines, follow stringent regulatory requirements, and respond to the needs of multiple sponsors. Day-to-day reporting requirements can drain valuable staff time and increase the risk of error, all while distracting from bigger goals of accelerating product development and ensuring compliance.
Signals Notebook, a comprehensive electronic laboratory notebook (ELN) solution from Revvity Signals Software, helps CROs enhance their productivity. But many users don’t realize they can get support to develop customized workflows that address specific pain points in their processes.
Recently, a global CRO specializing in pharmaceutical research and manufacturing partnered with the Revvity Signals team to streamline the process for a core deliverable—creating the certificate of analysis (COA), a key document delivered to every customer to validate results and demonstrate regulatory compliance. Through close collaboration, Revvity Signals provided a custom automation solution that reduced manual work, minimized errors, and freed up staff for higher-value tasks.
Manual Process
- Once all the result is received, user can create the manual COA as per various formats. Maintaining Consistency of the Report might be a challenge.
- Review of COA is mandatory as it is having human intervention and might be having some transcription error.
- Manipulation of data and result is possible.
- User needs to dedicate time and efforts to collects the data, prepared the report and get it approved.
- Can we benefit for small number of volume for generating a COA Report.
Automatic Process
- Result from Analytical department needs to be shared in a format that can be read and CoA can be generated automatically.
- Review cycle is not mandatory on this as it will be system generated based on the parameters defined by admin and validated before use on the production system by the users.
- No manipulation of the result and data in the COA.
- In automatic, very minimum effort and time is required to generate a report.
- It is beneficial if high volume of COA report is generated. It is scalable.
Automating COA Reports
Known for providing fully integrated drug discovery solutions—combining synthetic and medicinal chemistry, biology, and AI technologies—the CRO supports pharmaceutical clients from early research through commercial-scale manufacturing. With a strong emphasis on innovation, regulatory compliance, and global delivery, the organization aimed to optimize its customer-facing operations.
Previously, analytical leads manually compiled and reviewed data for each COA. The process was time-consuming and vulnerable to errors and inconsistencies, especially as the volume of work increased. Recognizing the inefficiencies, the CRO sought to automate COA report generation, to enhance accuracy, reduce turnaround times, and maintain a high standard of service for its clients.
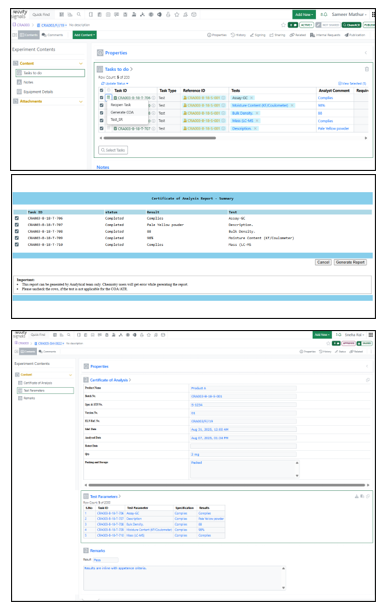
To address this need, the Revvity Signals customer success team conducted requirement-gathering sessions and refined the solution iteratively in close collaboration with the CRO’s stakeholders. They then developed the automation script and configured analytical workflows within the Signals Notebook platform. Their approach treated the CRO as a true partner, ensuring that the final product solved a real-world bottleneck the staff faced every day. The result was a fully integrated COA automation tool that fit seamlessly into the Signals Notebook platform that scientists were already comfortable using.
Outcomes: Less Risk, More Efficiency
Since the rollout of the automation tool, the CRO has reported faster turnaround, fewer errors, and a reduced manual burden on scientists. Staff can now generate COA reports with just a few clicks, improving both internal efficiency and client responsiveness. Feedback has been overwhelmingly positive, as users find the solution easy to use and highly reliable.
Takeaway: Customized Support Solves Persistent Pain Points
Whether it’s automated COAs or other recurring tasks, customized support from Revvity Signals can help solve operational pain points like preparing time-consuming but important paperwork. In this case, a strong partnership enabled the customer success team to thoroughly understand how to help the CRO automate the creation of the COA reports.
The model is scalable and adaptable, so the CRO can lean on the Revvity Signals customer success team to add more automation or solve new challenges that may arise in the future.
Building on the project’s success, the team is already exploring further enhancements such as expanded reporting capabilities and integration with other research documentation systems. There is also interest in extending similar automation to additional departments and workflows. As teams continue to eliminate even more manual processes, they will reclaim more time that they can spend on high-value scientific work.

End-to-end workflow support across scientific disciplines.

The Standard for Chemical Drawing.

End-to-end Clinical Data Science Platform.
