Risk Based-Monitoring
Leverage data-driven insights to uncover and manage key trial risks
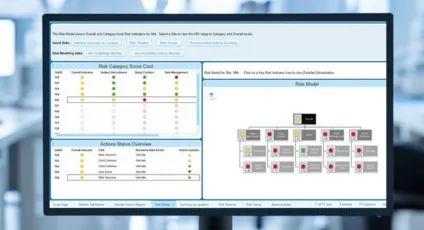
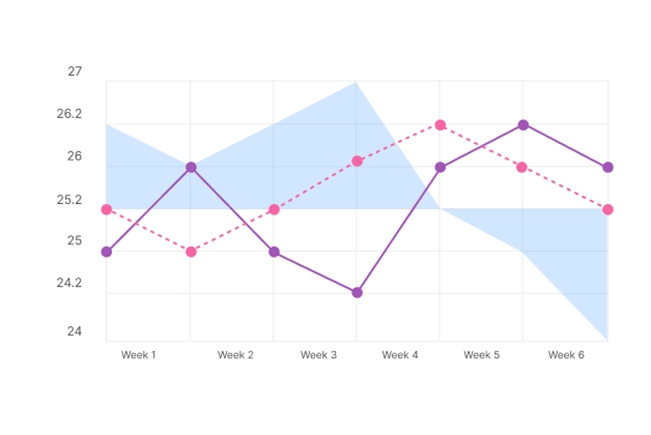
The Revvity Risk-Based Monitoring Solution provides analytics and visualizations to focus resources on high impact trial risks to ensure effective risk and data management


Scale the Delivery of Spotfire Analytics
Spotfire is an easy-to-use analytics platform that helps clinical researchers quickly uncover insights from clinical trial data. Spotfire easily accesses analysis ready data in Signals Clinical to enable self-service clinical analytics at biopharmas and CROs of all sizes globally.
Resources
Request a demo, or contact our experts to learn how Clinical Analytics can solve your risk-based monitoring challenges.