PROTACs® , Molecular Glues and more: How Informatics Helps Target the Undruggable
Learn about techniques such as Protacs and molecular glues. Explore Revvity's blog for more insights about how researchers are targeting the undruggable
In this blog post, we use the term ‘PROTAC’ as the common abbreviation for the therapeutic modalities encompassed by PROteolysis TArgeting Chimera. PROTAC® is a registered trademark of Arvinas.
Drugging the Undruggable: The Frontier of Drug Discovery
Conventional wisdom is that approximately 85% of the human proteome was historically deemed undruggable, meaning the proteins cannot be pharmacologically targeted with small molecule drugs.
The traditional pharmacological approach is to inhibit a strategic target protein within a biological pathway affecting disease progression. However, it is often challenging to design a selective and specific small molecule drug for every protein target. A more recent and effective approach has been instead to flag a protein for degradation within the cell. This approach is called targeted protein degradation.
The number of historically undruggable targets available translates into immeasurable opportunities for protein degradation approaches. Consider tumors – large percentages of cancers contain specifically mutated oncogenes. The ability to directly target those mutations could be game-changing for cancer treatments.
Understanding the Targeted Protein Degradation Market
Among new modalities for pursuing undruggable spaces, targeted protein degradation has attracted both attention and substantial pharma industry investment in recent years. One class of new drugs in the protein degradation space is called PROTAC®, which stands for PROteolysis Targeting Chimera.
These PROTACs are molecules composed of three parts, and each part could be counted as a small molecule itself. There are two different binding domains connected by a linker molecule. The fact that the PROTAC molecule has three different parts suggests that researching it is at least three times more complicated than conventional small molecules.
While PROTACs are by far the most investigated protein degradation modalities, two decades of research in the field has yet to yield an approved drug. Recently launched clinical trials are showing promise, but research needs to accelerate to create sufficient pipeline value for pharma companies to keep pursuing the complex and expensive lab work involved.
Defining PROTAC®
Proteolysis targeting chimeras (PROTACs) are heterobifunctional small molecules which induce selective proteolysis to remove target proteins by binding them with high selectivity on one end. This side is known as the warhead. The other side binds specifically to a ligase enzyme flagging the target protein, which is now bound to it by the PROTAC for degradation. Between both sides, the PROTAC molecule needs a linker, connecting the two active sides.
PROTACs latch on to proteins which lack the active sites typically required to bind small molecule drugs. This enables ubiquitin-mediated degradation and switches a target from undruggable to druggable. In other words, PROTAC attaches to a target protein and brings this target protein close to special cellular machinery called the proteasome. The proteasome acts like a garbage disposal for proteins and breaks the target protein down into smaller pieces that can be either reused or eliminated by the cell. In theory, this mechanism could be used to treat a disease by eliminating proteins involved in disease pathways.
PROTAC in Action
ARV-110 is a first-in-class oral PROTAC® protein degrader targeting the androgen receptor (AR) in men with metastatic castration-resistant prostate cancer (mCRPC). Androgen receptor signaling has been clinically-validated to play a role in the progression of mCRPC, which made ARV-110 a good test case for PROTAC® in early clinical trials.
Current drug treatments seek to inhibit AR, while ARV-110 aims to destroy it – potentially preventing prostate cancer from transitioning to a metastatic state. How? According to Chemical & Engineering News:
“One half of the PROTAC molecule binds to a protein of interest while the other half latches on to an E3 ubiquitin ligase. The E3 ligase then forms a complex that tags the protein of interest with ubiquitin moieties, which in turn signal that the protein should be sent to the proteasome for degradation.”
ARV-110 is currently in Phase 2 clinical trials.
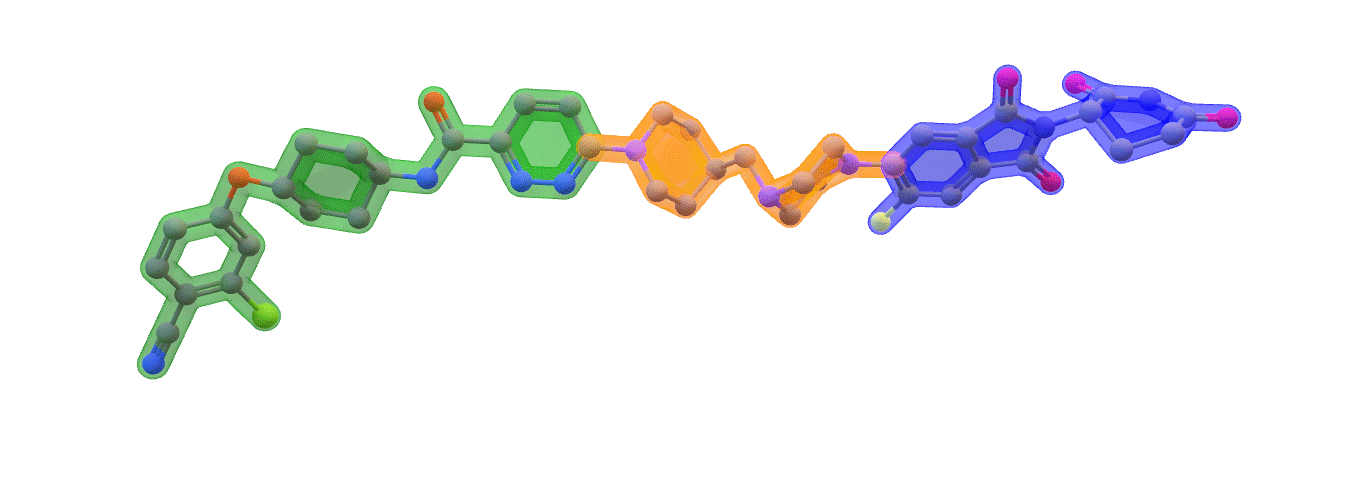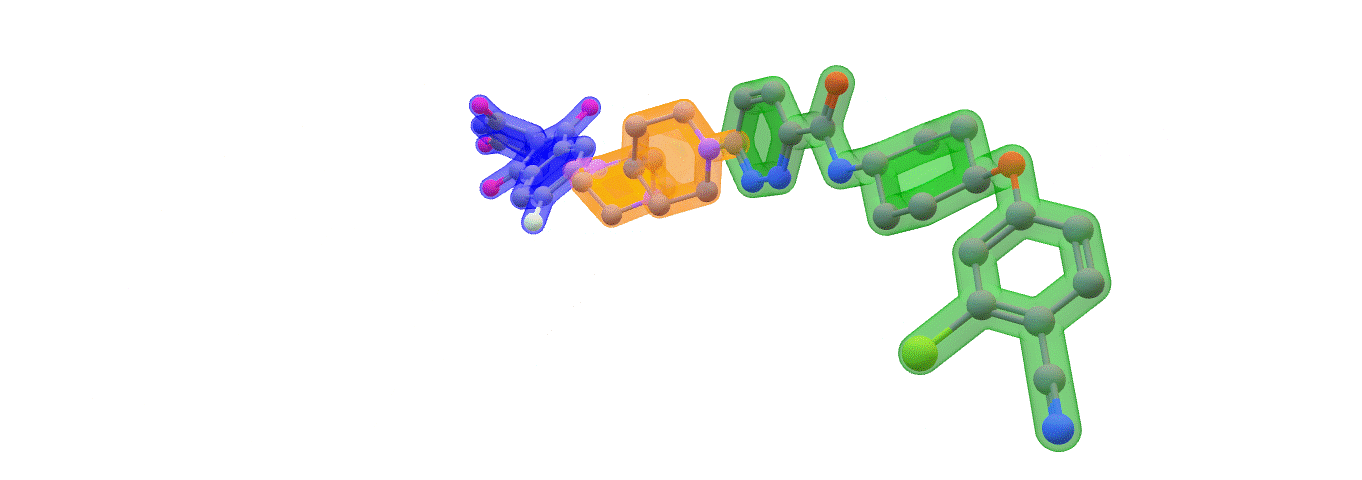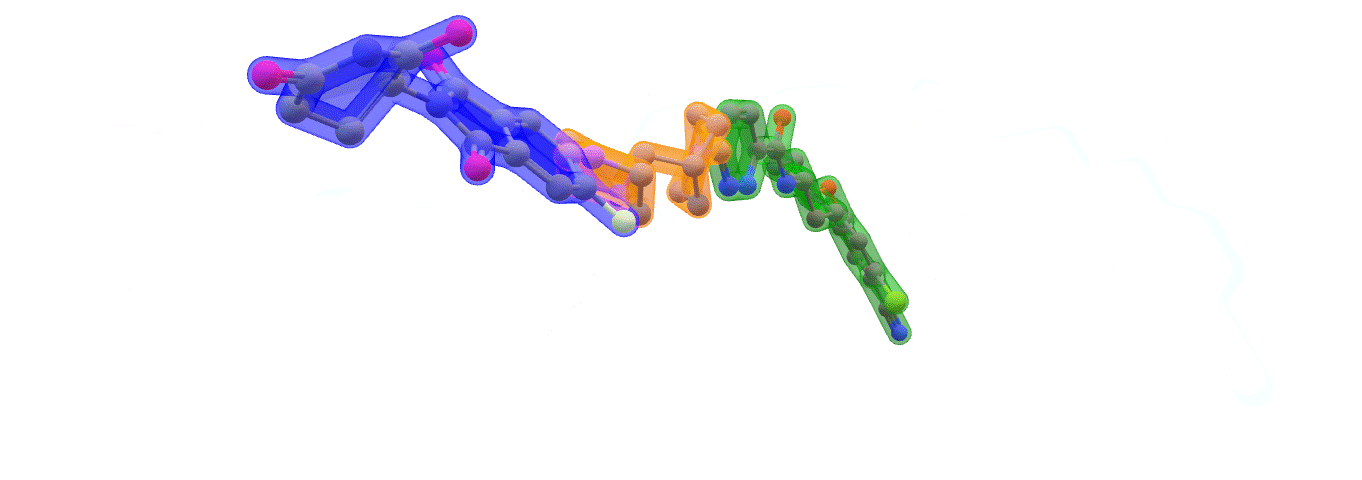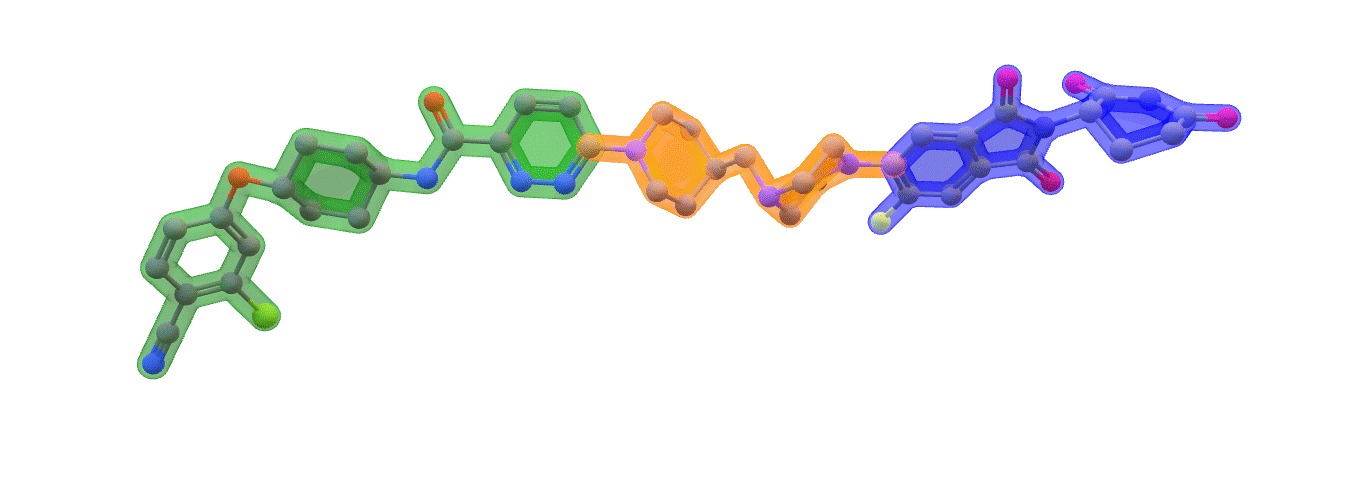
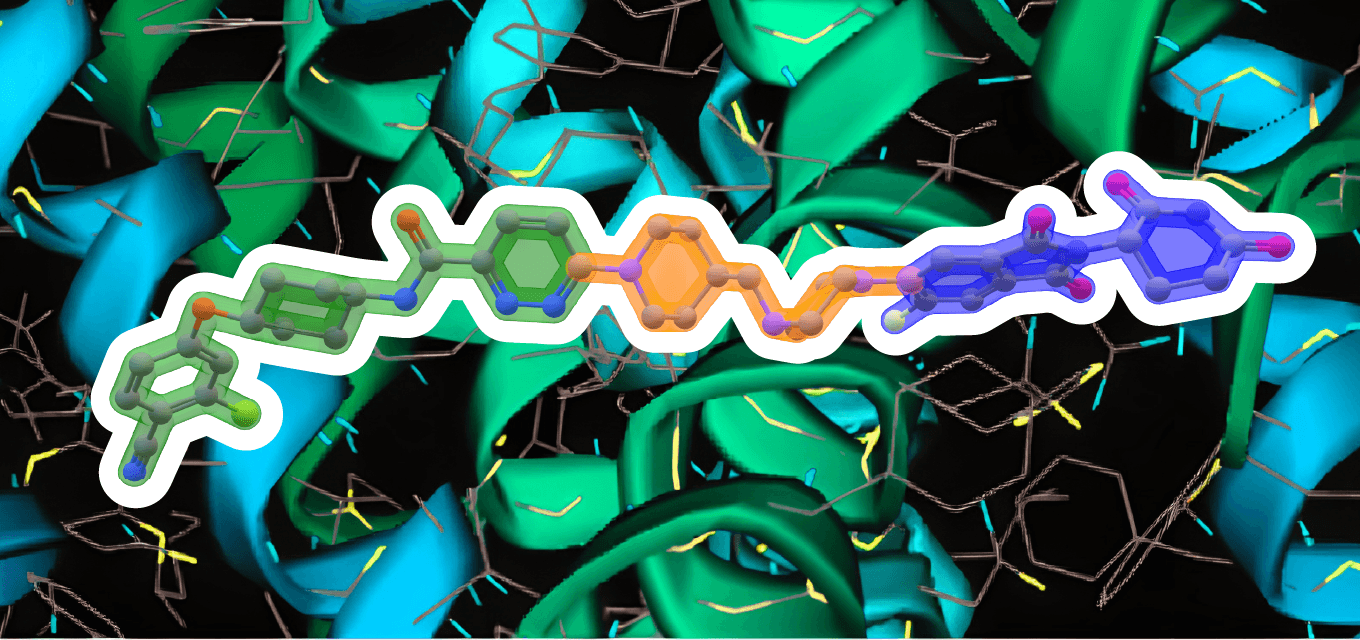
Image: ARV-110 (Bavdegalutamide) Warhead – green. Linker – orange. E3 binding – blue. Made with ChemOffice22
Building Upon Targeted Protein Degradation (TPD) Technologies
PROTAC is just a single modality in the targeted protein technology field. Others have emerged which may address some of PROTAC’s shortcomings. From Targeted Protein Degradation Represents a Promising Therapeutic Strategy:
“[E]merging new degradation technologies could significantly expand the variety of targets that may be selectively degraded. Some of them use a similar mode of action: TRAFTAC and molecular glue degrade proteins through the proteasome like PROTACs. On the other side, some technologies exploit a second major degradation pathway: LYTAC, AUTAC and ATTEC involve the lysosome degradation pathway.”
PROTAC was discovered back in 2008, but it is only in the last few years that there has been a rapid uptick in interest in TPD. Other technologies and modalities have since emerged, including:
- Molecular Glues
Molecular glues are monofunctional degradation activation compounds (monoDACs), unlike PROTAC® technology’s reliance on bifunctional degradation activation compounds (biDACs). Because MonoDACs tend to be smaller molecules than biDACs, they have the potential to overcome bioavailability and compliance with drug development guideline issues typical of PROTAC. -
TRAFTAC
TRAnscription Factor TArgeting Chimeras (TRAFTACs) are heterobifunctional chimeric oligonucleotides and are a type of molecule that can help regulate gene expression in cells. TRAFTAC targets transcription factors to help control when a gene is turned on or off.
- ATTEC
An AuTophagosome TEthering Compound (ATTEC) is a bifunctional molecule capable of binding simultaneously to a target protein as well as the phagophore protein LC3. An ATTEC helps promote fusion of the autophagosome with the lysosome to the target protein, enabling the two structures to work together to degrade the target protein. - AUTAC
The AUtophagy-TArgeted Chimera (AUTAC) consists of a specific binder for an intracellular target of interest and a guanine tag connected by a flexible linker. One part of the AUTAC binds to the unwanted protein while another part helps to bring the protein to the autophagy cell machinery for degradation. - LYTAC
Designed specifically to target cell membrane-bound and extracellular proteins, LYsosome-TArgeted Chimeras (LYTACs) make use of the lysosome degradation pathway by recruiting proteins to lysosome-shuttling receptors located at the cell surface.
TPD Research: Leveraging Informatics
Targeted protein degradation research is complex and multidisciplinary. The volume of data from disparate sources such as HTS, HCS, DMPK-PKPD and various other assay types is massive. Detailed data capture, centralization, standardization, and analysis is necessary to drive rapid understanding and discovery.
Scientists must have the essential tools necessary to access, combine, manage, share, and analyze data from in vitro assays and in vivo studies. TPD-specific workflows can help break down data silos, enhancing collaboration and accelerating drug discovery research.
Revvity Signals OneTM
Revvity Signals One covers all aspects of the necessary wet-lab research for Targeted Protein Degradation researchers to investigate PROTACs - including the bifunctional nature of PROTAC which often leads to pharmacological challenges.
Signals One combines functionalities enabling the development of new undruggable targets - from assay development to molecular biology and beyond. TPD research workflows encompass assay development, assay execution, high throughput screening, high content screening, molecular biology, bioprocess research, screening sciences, in vitro/in vivo/in silico analysis, bioanalytical, and even elements of traditional chemistry.
Signals One consists of Signals Notebook and Signals One Data Processing. Signals One Data unites assay development, low throughput to ultra-high throughput production assays, high content screening, and in vivo studies so users can work and analyze across all assay and screening data in a single platform. Signals Notebook is much more than an ELN solution as it is integrated with an inventory, material libraries, search, and analytics designed to orchestrate workflows, capture, and centralize data, and adhere to FAIR (Findable/Accessible/Interoperable/Re-usable) data principles.
How Does Signals One Break Down Data Silos?
Contemporary work on new drug modalities such as targeted protein degradation technologies highlighted in this blog require the input of many scientific disciplines, from biology to chemistry. With this greater need for project collaboration comes the need for better and more efficient information sharing.
Signals One permits effective work with biological data and data formats. Primers, Sequences, Vectors, Cell Lines and Plate information can all be included and examined in one place by anyone with appropriate permission access.
A typical TPD project can involve biological sequences, small molecule structures, experimental plans, assay results, and project summaries. This information needs to be accessible to all project members, and that’s why Signals One encodes every test, assay, and result with metadata tags – ensuring the data is open, searchable, and accessible to all.
Enterprise research organizations need seamless ways to make better tactical decisions while pursuing their strategic goal of finding new drugs for previously undruggable targets. Learn how Signals covers all aspects of wet-lab PROTAC research – or try it yourself! Visit the Signals One Interactive Workflow Demo.