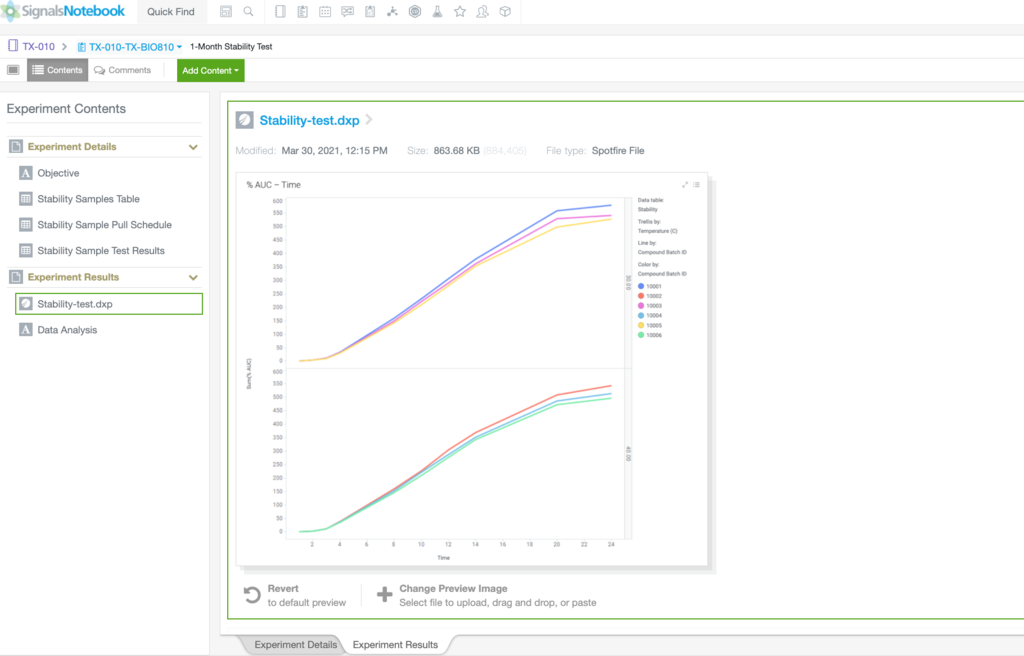
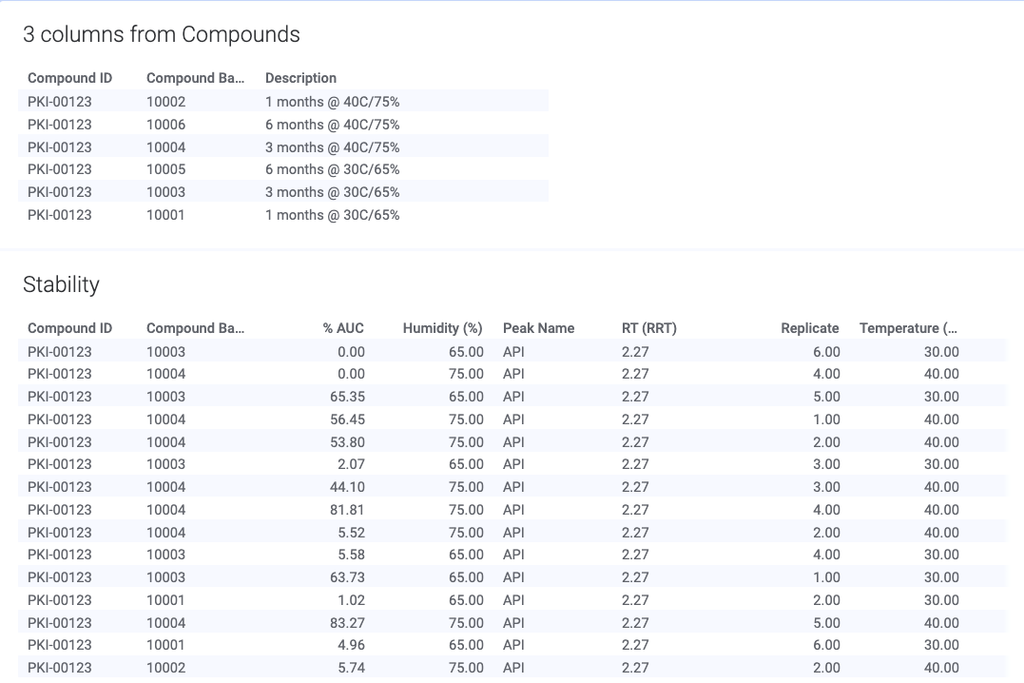
PHARMACEUTICAL DEVELOPMENT
Revvity Signals Software's solutions combine 35 years of scientific expertise to advance drug and treatment that accelerate discoveries
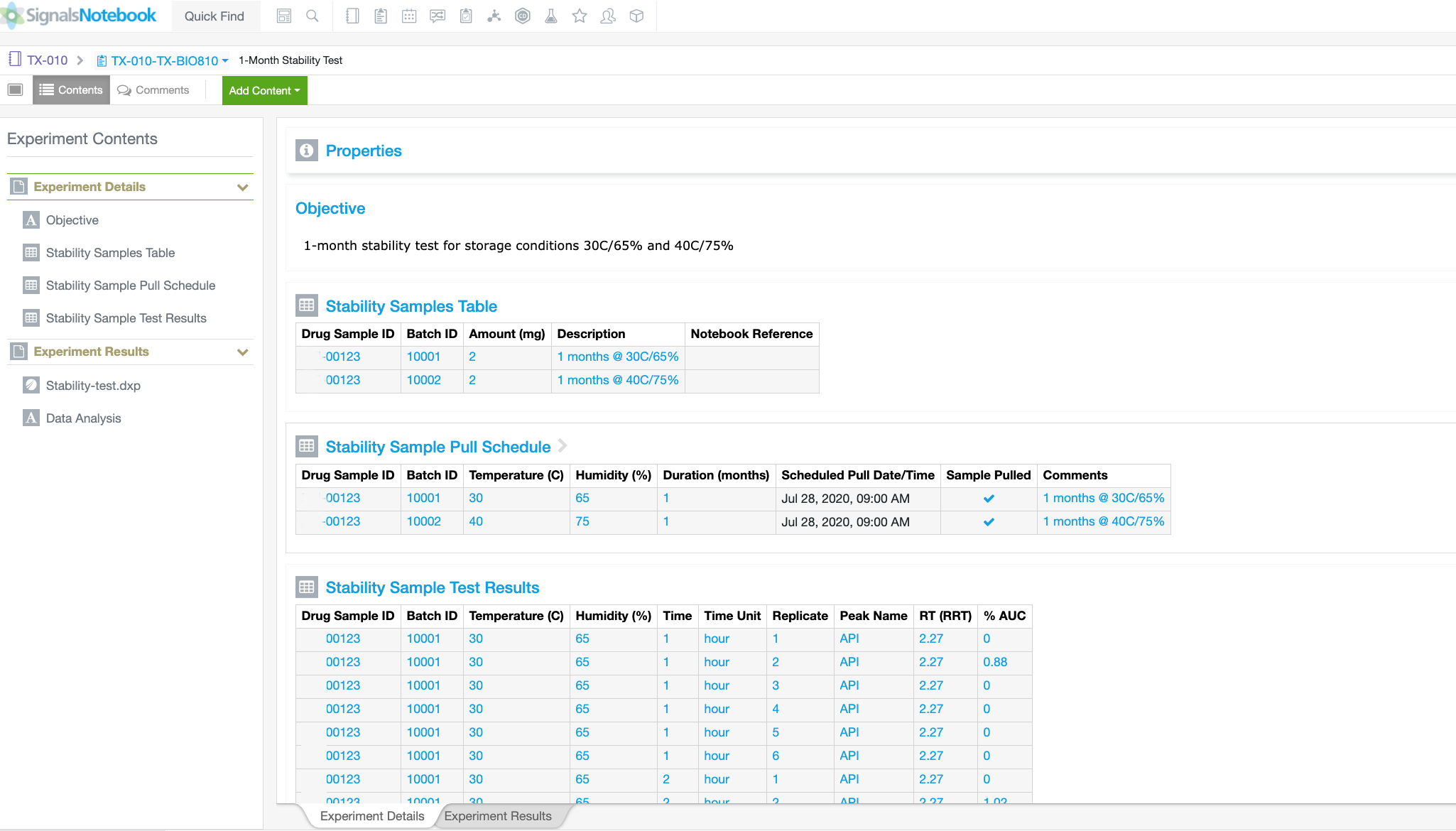
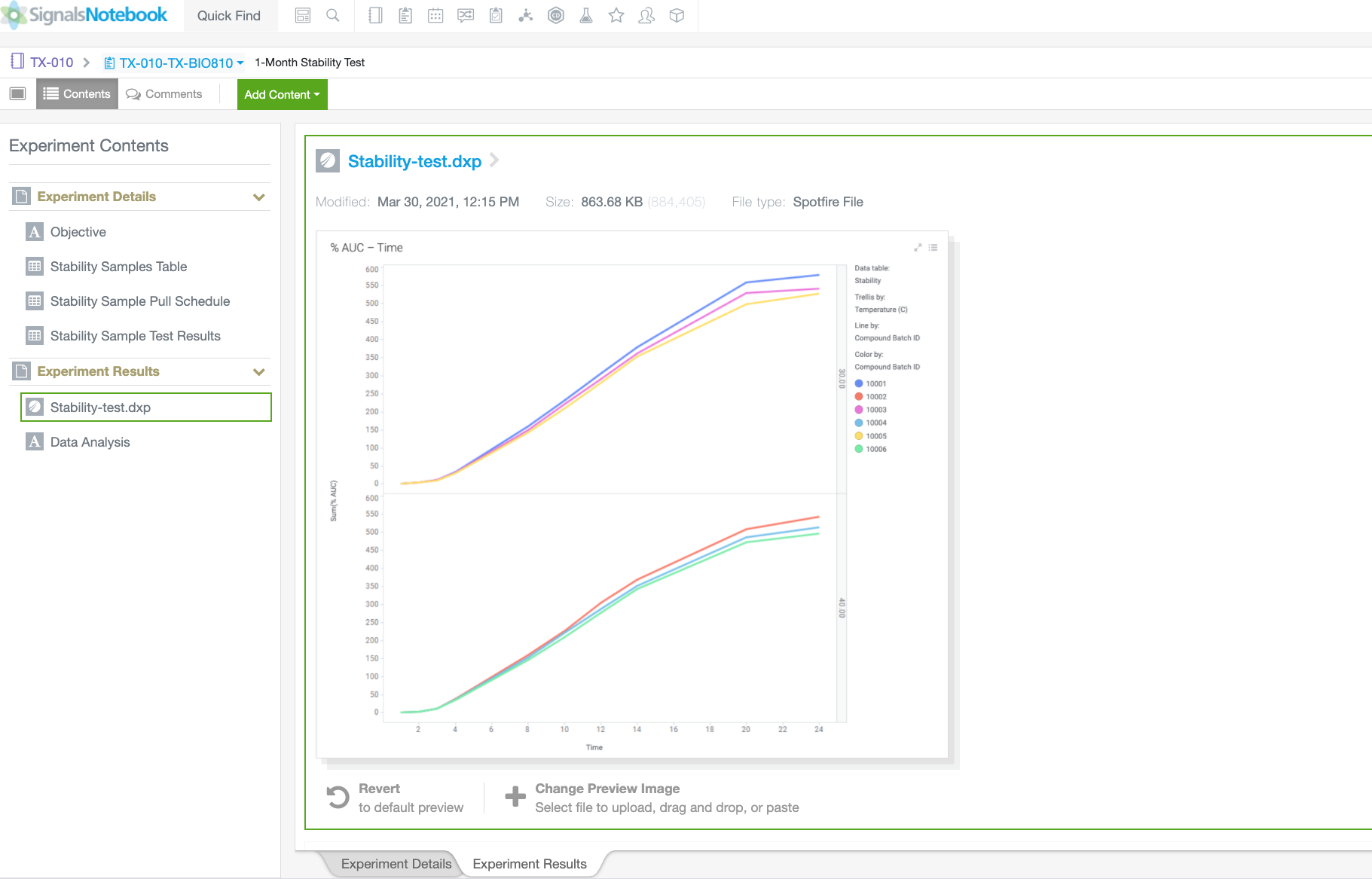
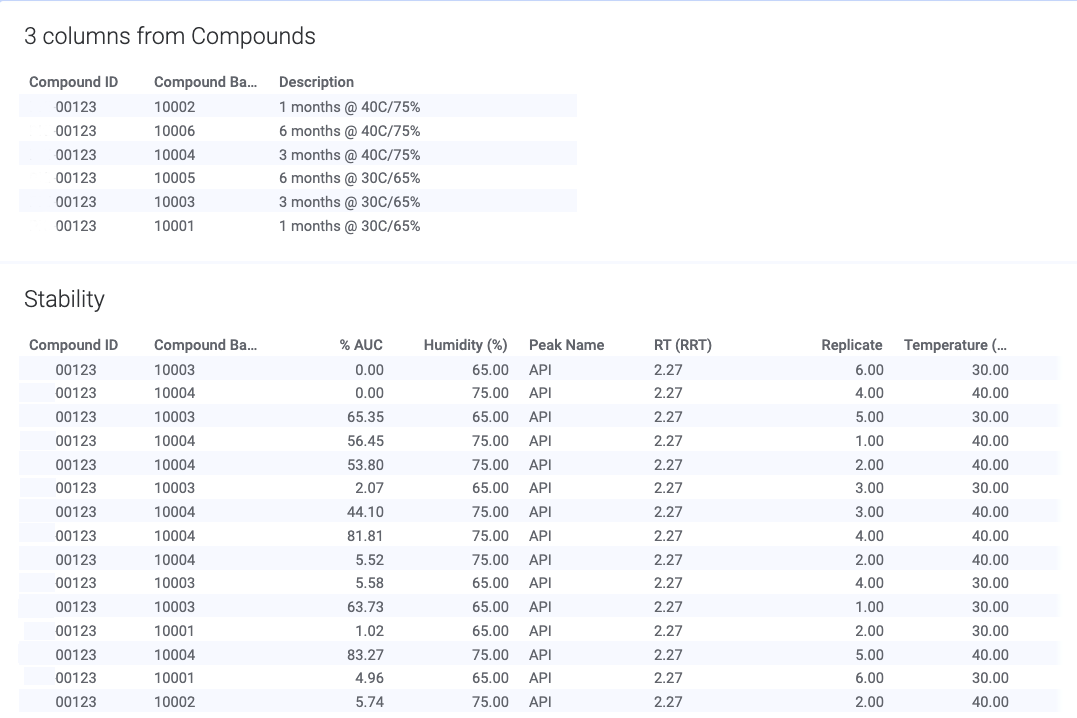
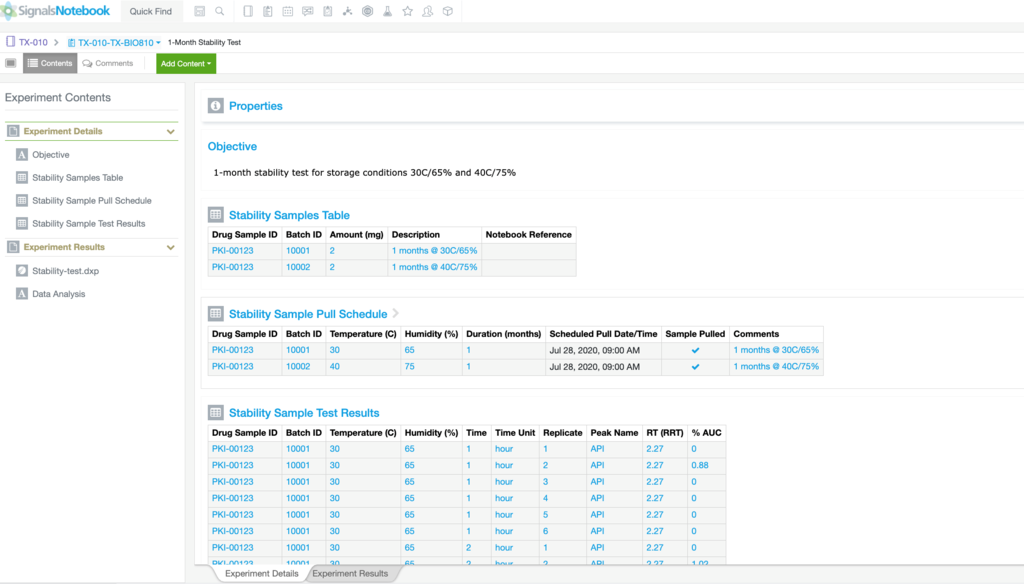
Automate workflows and gain insight across silos with our SaaS platform built for validation and compliance. See how formulation scientists and analytical chemists drive innovation while adhering to the recordkeeping rigor needed in Pharmaceutical Development.