Alzheimer’s Drug Discovery
Read about the latest Alzheimer's drug discovery research. Discover the innovative technologies being used in the fight against disease and offering new hopes.
Nearly 6.2 million Americans suffer from Alzheimer’s disease according to the Alzheimer's Disease Association. The World Health Organization reports that 55 million people globally suffer from Dementia, of which about 60-70% (33-39 million) are Alzheimer’s cases. Countless families have been touched in some way by this horrific, tragic disease.
New beta-amyloid antibody drug treatments
Alzheimer’s research has seen significant progress in recent years with the development of monoclonal antibody treatments which target the protein amyloid. Amyloid clumps or plaque development in the brain have been correlated with Alzheimer’s disease progression. Beta-amyloid protein plaques are believed to disrupt cell-to-cell communication in the brain, causing inflammation resulting in brain cell death. Research in the development of anti-amyloid beta antibody drugs has received significant attention over the last few years.
The following discussion provides some context on both early advances and recent significant progress in the field of Alzheimer’s drug research. This research has led to the development of therapeutics such as gantenerumab (Roche and Genentech)2, but many of these treatments have not progressed to the clinic as they failed to demonstrate a delay in cognitive decline in patients.
While early beta-amyloid antibody drugs haven’t shown as much progress as initially hoped, a recent antibody-based drug targeting the amyloid beta A4 protein is showing promise has recently received accelerated approval by the FDA.
Early Therapeutic Advances in Alzheimer’s Disease: Aducanumab (Aduhelm)
Beta-amyloid antibody drugs such as aducanumab (Aduhelm) gantenerumab and BAN2401 bind to soluble and insoluble amyloid beta aggregates. Aducanumab (Biogen’s Aduhelm) is a recombinant human antibody derived from a human blood lymphocyte which received “accelerated approval” from the FDA. It initially showed some promise, but its effectiveness in suppressing cognitive decline in Alzheimer’s needs further evaluation. It has also been found that there needs to be additional assessment of some identified adverse effects, such as brain swelling (ARIA-E edema/effusion) and bleeding (ARIA-H, cerebral microhemorrhages).
Recent Significant Progress in Alzheimer’s Drug Research: Lecanemab
Many of the initially developed beta-amyloid antibody treatments were halted due to significant side effects (including toxicity, brain swelling and bleeds) or limited effectiveness. But a newly approved drug, Lecanemab, shows promise.
Lecanemab’s development is significant because it is the first time that an amyloid antibody has been shown clinically to slow significant cognitive decline progression in Alzheimer’s patients. It has been reported to slow the rate of cognitive decline by 27% in controlled clinical studies. In early January 2023, Lecanemab was granted accelerated approval by the FDA for early-stage Alzheimer’s disease, though some drug-associated safety concerns remain.
The drug, developed by Biogen and Eisai, is an antibody which targets the amyloid beta A4 protein. It binds to amyloid beta protofibrils and is an anti-amyloid beta protofibril humanized monoclonal antibody.
Progress in Alzheimer’s drug discovery and development has historically trailed other disease states. But chemical communication software such as ChemDraw can help scientists accelerate research and bring drugs to market which improve health outcomes.
Leveraging ChemDraw, Chem3D, HELM and Biologics Visualization in Alzheimer’s Drug Research
Revvity Signals’ tools ChemDraw and Chem3D can facilitate the examination and study of these antibody-peptide interactions to enhance anti-amyloid antibody design. The introduction of HELM (Hierarchical Editing Language for Macromolecules - i.e., biomolecule structure representation) notation enables representation of modified peptides in ChemDraw with molecular visualization using Chem3D.
Where the size and complexity of the small-molecule and sequence-based information would ordinarily be impractical if drawn in software by hand, with HELM notation a scientist can think in terms of protein or nucleotide sequences. The software translates the sequence in terms of atoms and molecules to achieve a three-dimensional rendering (see Figure below).
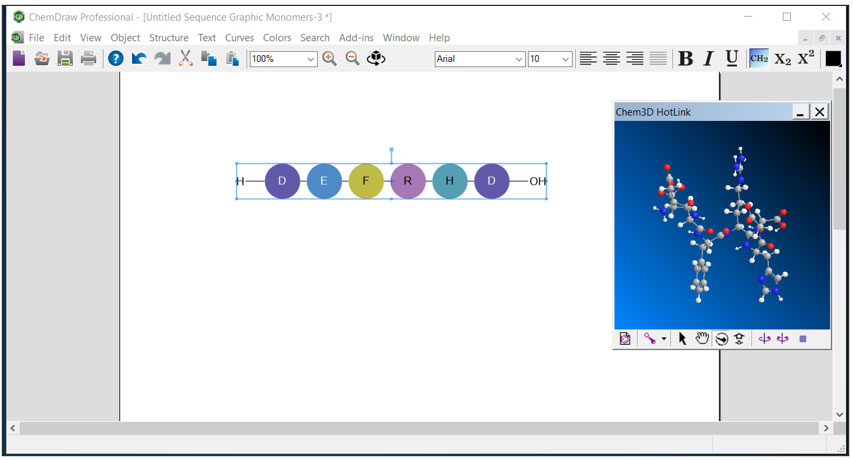
Easily Visualize Real World 3D Structures
Chemical structural information regarding the binding of the amyloid beta protein or peptide to the antibody Lecanemab has not yet been released in the global Protein Data Bank (PDB). But chemical structural information is available for several of the other related beta-amyloid antibody drugs with amyloid peptides, including gantenerumab, PDB:5CSZ and aducanumab PDB:6CO3.
The structure of Aducanumab is available in the protein data bank (PDB:6CO3 aducanumab antibody - amyloid beta peptide complex). It is shown below as represented in Revvity Signals software Chem3D. Aducanumab binds a linear epitope of the beta-amyloid peptide formed by amino acids 3-7 of the peptide.
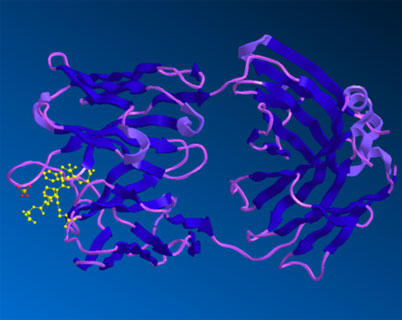
Figure A is the structure of the entire antibody Fab bound with the amyloid beta peptide.
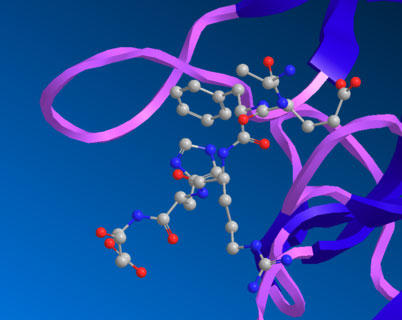
Figure B is a detail of the binding interface between the aducanumab antibody Fab fragment and the interaction with the amyloid beta peptide.
Other similar antibodies such as gantenerumab (PDB:5CSZ) have different binding modes with the amyloid beta peptide, as shown in the figure below.
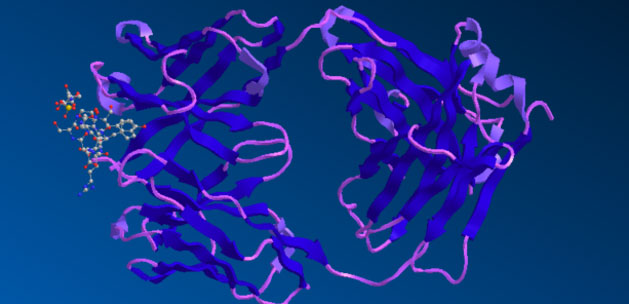
The solved crystal structure of the Fab fragment of aducaumab bound to its epitope peptide shows that aducanumab binds to the N terminus of the beta-amyloid peptide. It binds a compact epitope in a shallow pocket on the antibody surface.
This effective amyloid peptide binding to the aducanumab antibody is different than the peptide binding to the other less effective antibody, ganternerumab, as shown in these figures.
Aducanumab binds to the amyloid beta residues in an extended conformation, and the conformation and orientation of the bound peptide are different than other antibodies that recognize amyloid beta N-terminal peptide epitopes. Residues 2-7 of the beta-amyloid peptide bind the aducanumab antibody bound in an extended conformation with residues Phe 4 and His 6 of the amyloid beta peptides, making primary contact with the antibody fragment.
Peptide, protein, and antibody sequences can all be effectively manipulated and stored for experimental design within Signals Notebook (shown below). The Revvity Signals Notebook platform – Signals Notebook, Vitro Vivo, and Inventa can keep track of modified antibody and peptide design, synthesis, manufacturing steps & procedures, animal testing, and assay results.
Learn how the Signals Platform, using the ChemDraw HELM format, facilitates the development of antibodies and biologics.
Resources
(1) Structural and kinetic basis for the selectivity of aducanumab for aggregated forms of amyloid-β Joseph W. Arndt, Fang Qian, Benjamin A. Smith, Chao Quan, Krishna Praneeth Kilambi, Martin W. Bush, Thomas Walz, R. Blake Pepinsky, Thierry Bussière, Stefan Hamann, Thomas O. Cameron & Paul H. Weinreb. Scientific Reports, (2018) 8:6412, DOI:10.1038/s41598-018-24501-0
(2) Gantenerumab: A Novel Human Anti-Aβ Antibody Demonstrates Sustained Cerebral Amyloid-β Binding and Elicits Cell-Mediated Removal of Human Amyloid-β Bohrmann, Bernda;, Baumann, Karlheinza , Benz, Jörga , Gerber, Francoisea , Huber, Waltera, Knoflach, Frédérica, Messer, Jürga , Oroszlan, Krisztinaa, Rauchenberger, Robert, Richter, Wolfgang F., Rothe, Christineb, Urban, Margitb, Bardroff, Michael, Winter, Michaela, Nordstedt, Christera, Loetscher, Hansruedia. Journal of Alzheimer's Disease, vol. 28, no. 1, pp. 49-69, 2012
(3) Lecanemab Confirmatory Phase 3 Clarity AD Study Met Primarily Endpoint, Showing Highly Statistically Significant Reduction of Clinical Decline in Large Global Clinical Study of 1795 Participants with Early Alzheimer’s Disease. News release. Biogen; September 27, 2022. Accessed November 2, 2022. https://investors.biogen.com/news-releases/news-release-details/lecanemab-confirmatory-phase-3-clarity-ad-study-met-primary