Signals Clinical
Faster, Smarter Clinical Data Review - Built to Scale with You
Signals Clinical™️is an AI-powered clinical data review solution that unifies data review, querying, and medical monitoring in one intuitive solution that adapts to your workflows. Whether accelerating your first clinical trial, managing a global study portfolio, or standardizing enterprise-wide oversight, Signals Clinical helps teams detect risks earlier, streamline review cycles, and unlock data-driven insights that improve trial outcomes.
Discover Signals Clinical
Learn how Signals Clinical facilitates the rapid delivery of deep insights into clinical trial data.

Transform Clinical Data Management
Learn how to harness AI and machine learning to transform clinical data management, increase understanding of clinical trial data, and improve trial execution and clinical strategy. Using natural language, study teams will be able to generate listings.
Signals Clinical Connect Webinar Series
Discover how Signals Clinical supercharges your clinical data management workflows with real-time insights, purpose-built visualisations, and seamless Spotfire integration.

Faster, Smarter Data Review with Signals Clinical™ + Spotfire®
The only clinical data solution that unites prebuilt trial visualizations with the flexibility to adapt to any study design. Why Teams Choose Signals Clinical™ + Spotfire®
- Start instantly: Get insights in minutes with out-of-the-box clinical visualizations—no coding required.
- Stay flexible: Build and customize dashboards in Spotfire to address the unique demands of your trial.
- Scale seamlessly: Standardize analytics across studies and portfolios for consistent oversight and governance.
- Act with confidence: Accelerate safety and operational reviews using real-time, audit-ready insights.
Streamline Clinical Development with Clinical Analytics
Clinical Data Review
Detect safety & data quality issues earlier with prebuilt clinical visuals and in-context queries. Fewer on-site visits, earlier risk mitigation.

Risk-based Monitoring
Prioritize sites and patients using risk signals, thresholds, and trend alerts across studies. Faster oversight, fewer delays.

Pharmacovigilance
Trace adverse events from signal to decision with listings, narratives, and audit trails. Faster case assessment, inspection-ready history.
Clinical Operations
Centralize KPIs (enrollment, cycle time, protocol deviations) for proactive actions. Shorter cycle times, fewer bottlenecks.

Resources
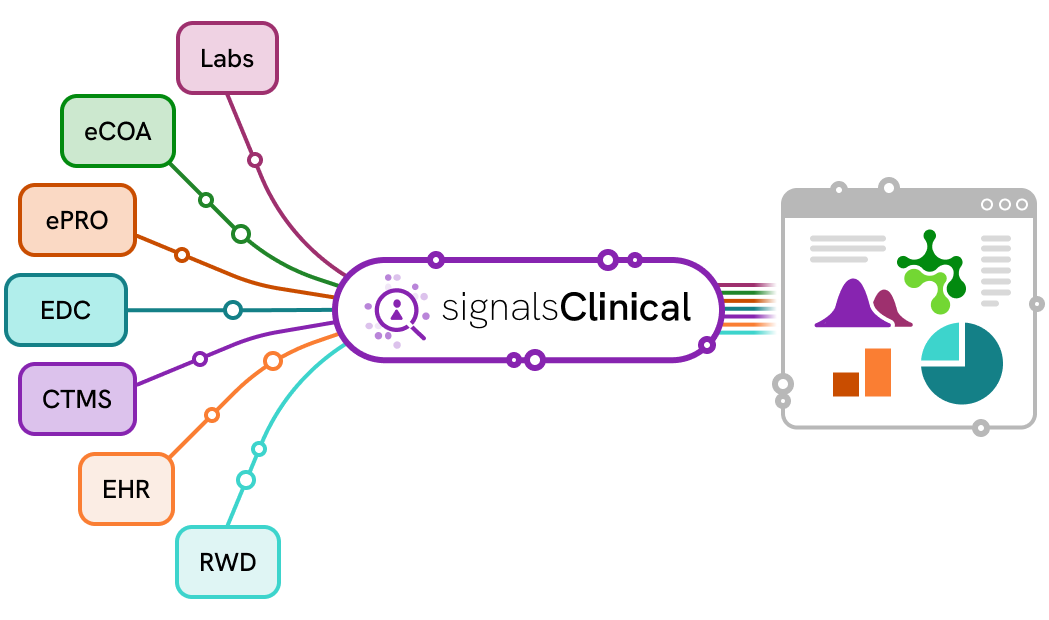
Signals Clinical™ is an end-to-end clinical data science solution that eliminates fragmented workflows and manual bottlenecks—centralizing review, accelerating access to clean, analysis-ready data, and ensuring traceable oversight across studies. It connects seamlessly with Spotfire® so teams can enhance their visualizations for custom analytics, delivering deeper insights, stronger oversight, and faster study decisions.
- Brochure: An End-to-End Clinical Data Science Platform
- White Paper: Powering More Efficient Clinical Development with AI and ML
- White Paper: Fomenting A Culture Of Analytical Excellence in Clinical Development
- Brochure: Clinical analytics solutions
- Blog: Smarter Clinical Data Review
- White Paper: Accelerating Clinical Data Review with Traceable Workflows
Frequently Asked Questions (FAQs)
What is Signals Clinical?
Signals Clinical is a software-as-a-service (SaaS) clinical data science solution designed to centralize and streamline clinical data review and medical review. It provides real-time access to all clinical trial data, enabling rapid loading of study data through reusable data maps and a simple, no-code interface for cross-study searches. By facilitating data review, medical monitoring, efficacy analysis, safety case analysis and signal detection (pharmacovigilance), and RQBM capabilities, Signals Clinical ensures comprehensive oversight of patient safety and trial data quality.
What EDC solutions does Signals clinical integrate with?
As an end-to-end clinical data science solution, Signals Clinical simplifies access to the clinical trial data needed for analysis from virtually any source, including EDC solutions Medidata Rave, Veeva Vault EDC, and many other sources.
What makes the Signals Clinical unique compared to other clinical analytics solutions?
Signals Clinical is the only solution that provides prebuilt standard clinical visualizations combined with the ability to build study or therapeutic specific visualizations in Spotfire. This facilitates rapid delivery of the insights clinical researchers need to inform critical study decisions.
Can Signals Clinical support both single-study and cross Study Analysis?
Signals Clinical is an end-to-end clinical data science solution that facilitates the delivery of insights for a single study or across multiple studies. Portfolio search allows you to select and filter datasets from multiple studies and then export the data needed for cross-study analysis.
What are the primary clinical research processes Signals Clinical facilitates?
Signals Clinical provides analytics and workflow support for clinical data review, medical review, efficacy analyses, safety case analysis and signal detection, and RBQM and operations.
What is the AI capability in Signals Clinical?
Signals Clinical features an AI-assisted Custom Listing Generator that translates natural language requests into precise, clinical data listings with clear audit trails-oversight made easy. It empowers data managers, monitors, and medical reviewers to access study data in minutes — without relying on programmers or external CROs.