Signals LabGistics™
Streamline your scientific workflows across the entire drug development lifecycle.
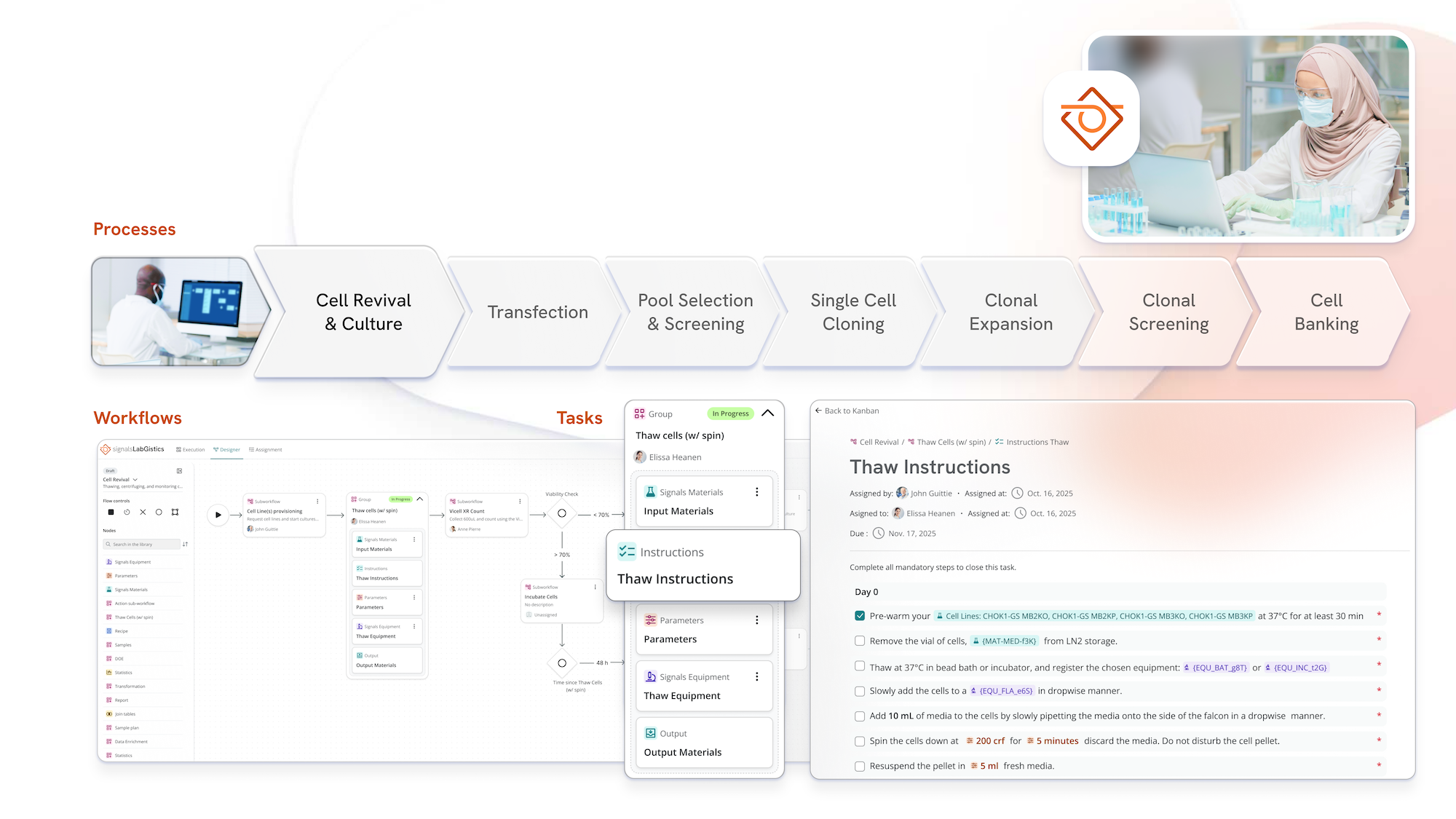
With Signals LabGistics, you can plan, execute, and monitor your work while ensuring your data flows smoothly from R&D through to manufacturing without the manual gaps, delays, or handoff frustrations slowing you down today.
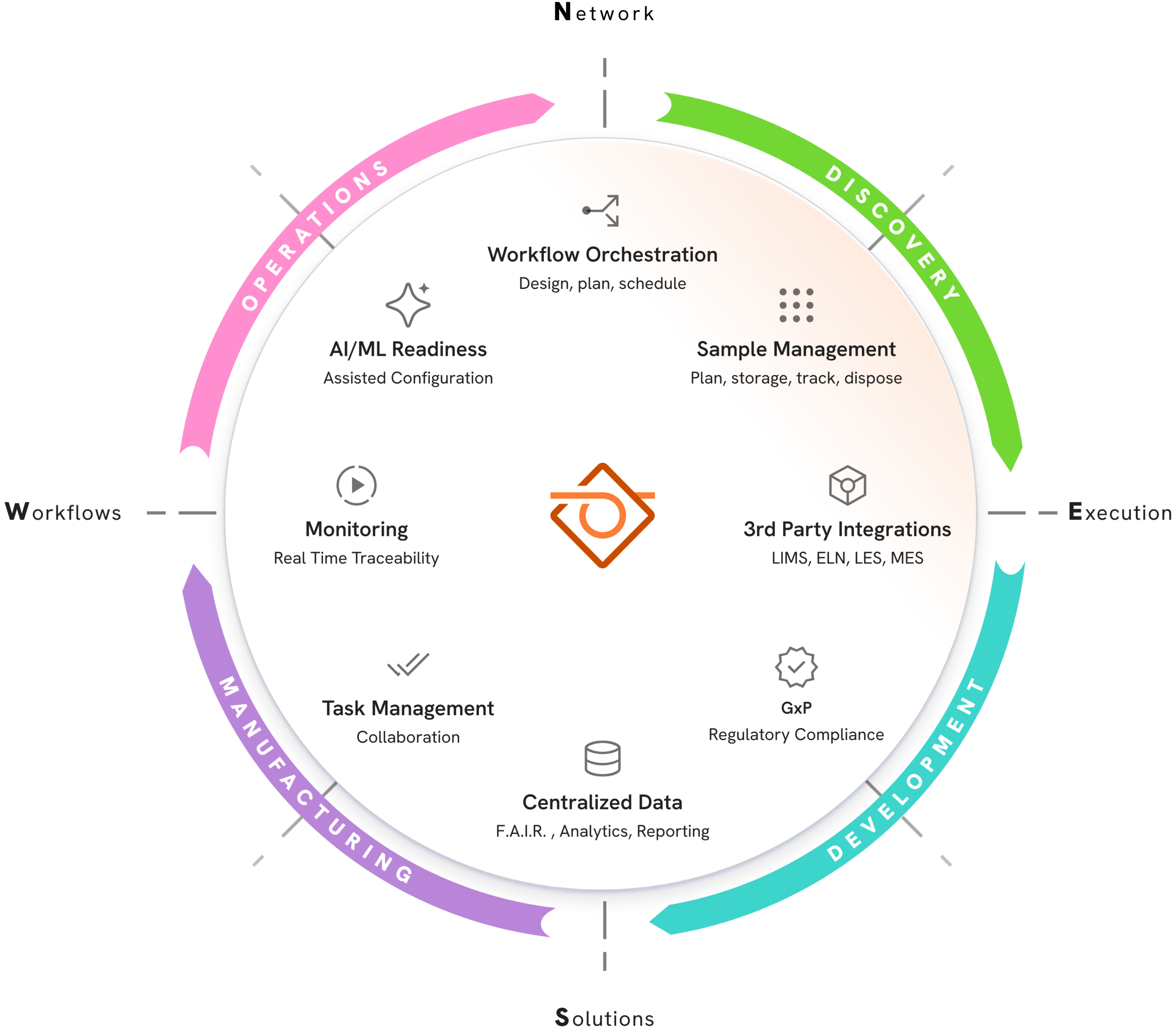
Create a Single Source of Truth for Scientific Operations
Signals LabGistics creates a structured environment that supports the design, planning, execution, and monitoring of workflows across diverse scientific fields.
Bridge workflow coordination gaps by eliminating email handoffs and manual transfers between teams.
Accelerate technology transfer through seamless handoffs between development and manufacturing processes.
Simplify regulatory compliance by supporting documentation and traceability in GxP environments.
Generate automated workflows using AI tools that convert SOPs and natural language descriptions into processes.
Security
Secure Data
Independently-verified best practices for storing and securing data.
Cloud
Built with secure cloud infrastructure on AWS.
Access Config
Permissions-based access means team members only see what they need to.
SOC 2 Certified
Verified compliance with industry-leading security and privacy standards.
Ready to Achieve Workflow Coordination with Signals LabGistics?
Get Early Updates
Streamline collaboration, reduce costs, and realize faster, more efficient drug development—all from this single, cloud-native solution.
Read Brochure
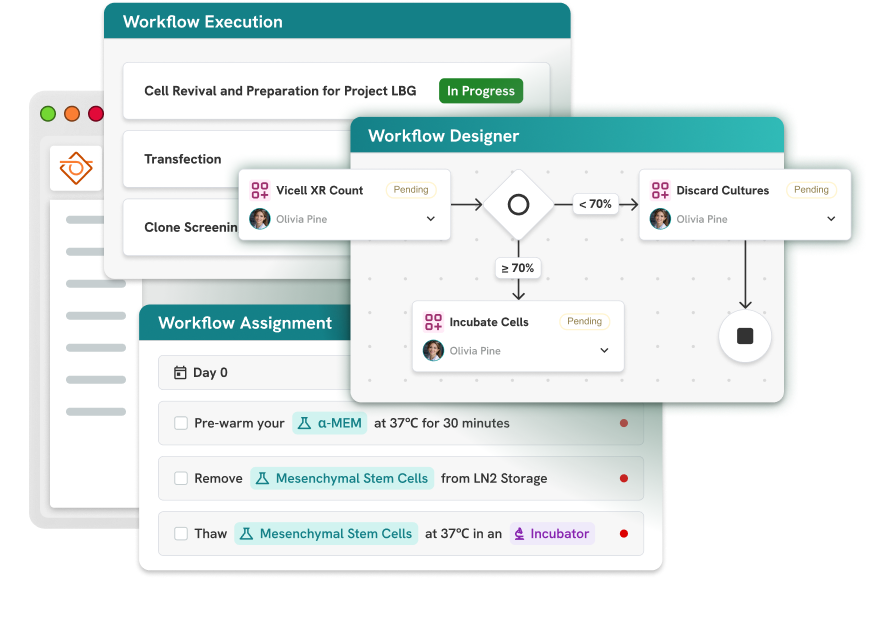
Learn more about three-tier workflow management—processes, workflows, and tasks—including scalable workflow execution and monitoring.
Keep up with the latest with guides, research, and insights.
Signals LabGistics FAQs
Ready to take the next step?
Stay up to date.
Want to keep up with all the upcoming Signals LabGistics news and product information?